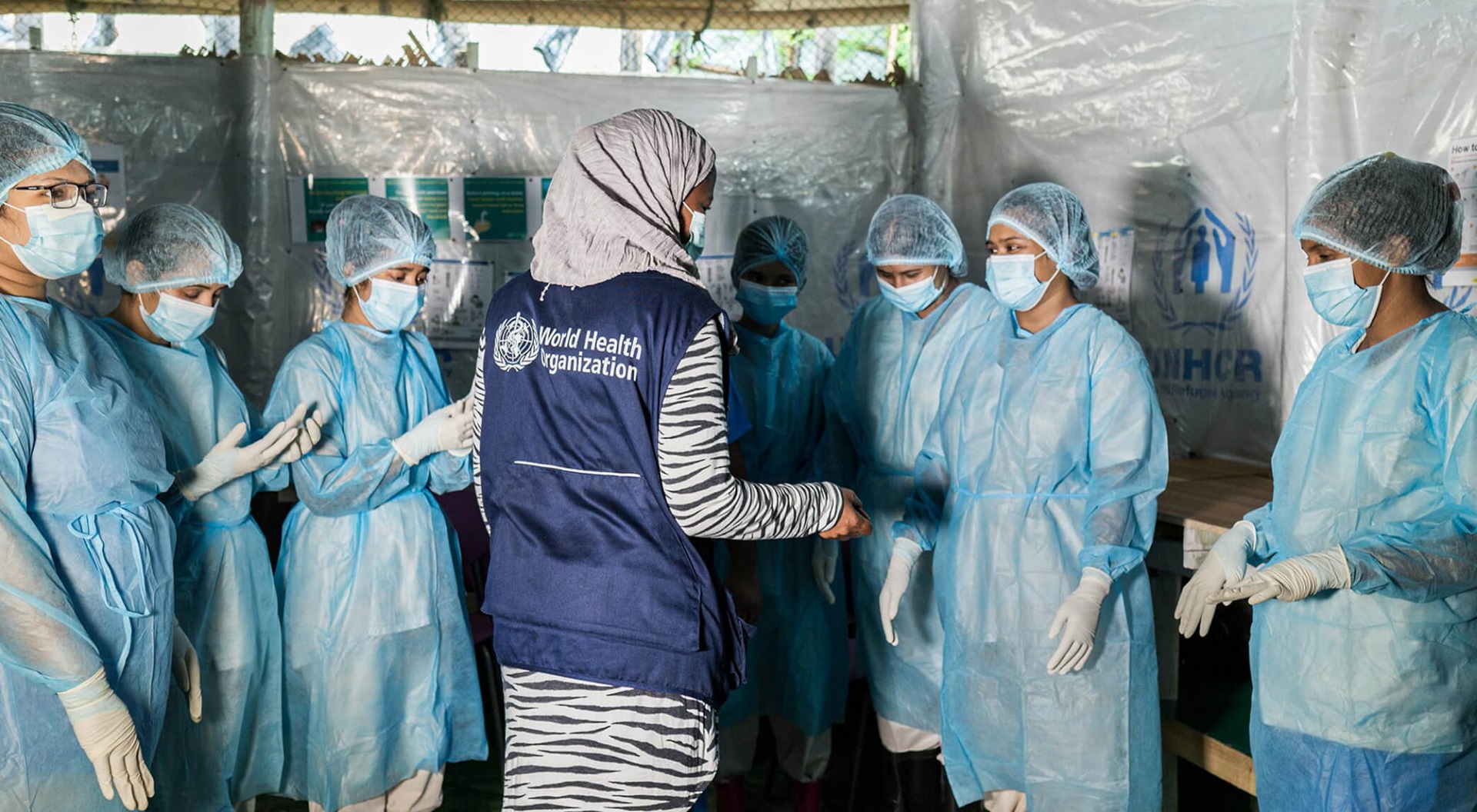
Responding to COVID-19 in 2020 presented both a challenge and opportunity for epidemic and pandemic prevention.
Epidemic and pandemic prevention applies to two broad categories of diseases. The first are known and established threats, such as cholera and yellow fever, for which the world has safe, effective strategies and countermeasures for prevention and rapid
response. The second group are high-threat pathogens for which the world has no countermeasures. This group includes known and hypothetical (“disease X”) infectious pathogens that might emerge via zoonotic spill-over or due to accidental
or deliberate release.
Prevention of and response to outbreaks of diseases in both groups require effective strategies and tools and effective stewardship and governance mechanisms for strategic stockpiling and equitable use of global goods such as vaccines and therapeutics.
In the case of both hypothetical disease X pathogens and known high-threat pathogens, an effective response also requires a global mechanism to set priorities, fund and accelerate research and development of medical and non-medical countermeasures
and a global mechanism for large-scale manufacture and distribution. All rely on a global network of knowledge, expertise and operational capacity and capability, with WHO and the WHO Health Emergencies programme at its centre.
The start of 2020 witnessed the emergence of SARS-CoV-2, which causes COVID-19, a zoonotic disease with pandemic potential. The world had only embryonic scientific and clinical understanding of the disease and no medical countermeasures. By the end of
2020, the world had:
- administered the first doses of the first generation of safe, effective vaccines;
- launched a global partnership, Access to COVID-19 tools (ACT), designed to accelerate discovery, development, production and access to effective vaccines, therapeutics and diagnostics on the basis of need;
- cultivated clear, constantly evolving understanding of the public health and social measures to control transmission of SARS-CoV-2;
- learnt how to treat COVID-19 more effectively;
- established and leveraged the new discipline of “infodemiology” to study how differential access to public health information (and misinformation) shapes public health outcomes; and
- developed new tools and strategies to track transmission of SARS-CoV-2 and detect variants of concern in a wide variety of settings.
None of these achievements was WHO’s alone, but all had WHO at their heart.
2020 was the year in which many of the platforms built and strengthened after establishment of the WHO Health Emergencies Programme in 2016 proved their worth, catalysing and operationalizing a science-led, evidence-based response to COVID-19, including
through new mechanisms and platforms for rapidly convening and working with expert networks and advisory groups such as the Strategic Advisory Group on Infectious Hazards, WHO collaborating centres and regional and national knowledge and expert platforms.
WHO published its first comprehensive guidance on COVID-19 on 10 January 2020 and subsequently published and continually updated guidance on every aspect of the public health response, tailored to every context and translated into training modules on
the OpenWHO platform, which were accessed more than 4 million times during 2020.
From the initial emergence of SARS-CoV-2, WHO and Member States leveraged many of the pandemic influenza capacities developed and strengthened over the past decade, including laboratory detection capacity at national influenza centres and sentinel surveillance
through WHO’s Global Influenza Surveillance and Response System and associated influenza surveillance systems. This also provided a valuable mechanism for sharing genetic sequence data on SARS-CoV-2, including for monitoring and analysing SARS-CoV-2
variants of concern. Expansion of these capacities globally will have benefits far beyond COVID-19 and should complement efforts to accelerate access to high-quality pathogen specimens and genomic data during outbreaks and an equitable benefit-sharing
scheme. The efforts include the Biohub initiative to build a global repository for rapid sharing of pathogens from a standardized collection, characterization and archiving of viruses, other pathogens and specimens to facilitate and accelerate development
of diagnostic tests and their evaluation for diseases of epidemic potential.
Global coordination of research and development for COVID-19 began in early January 2020 and was accelerated in February by the Global Research Roadmap, published as part of the WHO R&D Blueprint for Research in Epidemics. These activities were crucial
for the development, in record time, of new vaccines and diagnostics, which were then assessed and, when appropriate, granted emergency use listing by WHO to accelerate their adoption and use in countries. The Solidarity Trial for therapeutics was
the largest of its kind – truly global in scale – and the Unity Study protocols for serological surveys were used by countries of all capacities to understand how SARS-CoV-2 had and could spread in their populations. Building on previous
work and efforts to strengthen national capacities for research and regulatory governance during outbreaks and epidemics, WHO helped to ensure rigorous ethical review and oversight of COVID-19-related research in countries in all regions.
Through the ACT Accelerator, launched with partners in April 2020, WHO ensures equitable access to COVID-19 tools, including vaccines, therapeutics and diagnostics, by all populations around the world. In a future in which the world is prepared to prevent
and rapidly respond to epidemics and pandemics, rapid development of innovative tools such as vaccines and diagnostics must be complemented by an established, sustainable global mechanism to ensure that technologies are tested, manufactured and distributed
at scale, with an absolute commitment to equity to ensure that they fulfil their potential as global public health goods.
The speed and scale of the development of medical countermeasures to COVID-19 was a major success of 2020. WHO and partners also developed a new multidiscipline for emergency response, infodemiology, and infodemic management tools to better understand
how health information is used by communities, how it is translated into behavioural changes and therefore how best to intervene to improve health outcomes. During 2020, WHO’s infodemic management team developed the Early AI-supported Response with Social
(EARS) listening tool, which allows health decision-makers to view real-time analyses of narratives on public health topics that predominate on public online forums, in many countries and languages. The tool provides policy-makers with rapid, real-time
understanding of public concerns, so that they can adjust policy accordingly. Unlike other proprietary social listening tools, EARS is free of charge and can be used in contexts where budgets would not accommodate investment in infrastructure and software,
promising refinement of the response not only to COVID-19 but also to future health emergencies.
The race to develop and roll out tools and strategies to counter COVID-19 required repurposing of resources and capacity from some of WHO’s normative work; however, recent progress in combatting vaccine-preventable epidemic-prone pathogens was largely
maintained during 2020. Notable achievements include a significant increase in the supply of yellow fever vaccine, such that an estimated 48 million people were protected against yellow fever in Africa and the Americas by mass vaccination campaigns
during 2020. After initial disruption of global immunization activities, both outbreak response and preventive oral cholera vaccine campaigns were safely resumed throughout 2020, with 13 million oral cholera vaccine doses shipped to eight countries,
including almost 8 million doses for preventive vaccination. Progress was also made in developing tools and strategies to control outbreaks of Ebola virus disease and meningitis.
WHO will continue to work with partners to ensure that the response to COVID-19, and in particular the drive to strengthen national capacity for administering COVID-19 vaccines, is translated into accelerated progress in the prevention of epidemics and
pandemics. Although the approaches and tools used to control infectious diseases differ, they have several common requirements, such as a mechanism for allocating scarce resources, platforms to plan, coordinate and resource national control strategies
and integration into national health systems. Most control strategies apply to the same countries and regions, which are mainly low-capacity, fragile, conflict-affected, vulnerable settings. Tools developed during the COVID-19 response to plan, coordinate
and resource national action plans can be used in a new, integrated approach to epidemic and pandemic prevention that brings together disease-control programmes, national authorities, partners and donors working on common plans and shared platforms
to coordinate and support delivery of disease-control strategies for the benefit of the most vulnerable populations and to protect global health security. COVID-19 has also shown that clear benefits are to be gained from integrating influenza and
other high-threat respiratory pathogens such as coronaviruses (including SARS, MERS, COVID-19) into one platform in order to maximize investments in critical capacities. As challenging as the response to COVID-19 has been, the capacities built and
the lessons learnt during 2020 offer an opportunity to change the face of infectious disease prevention and control.
Pandemic Influenza Preparedness Annual Progress Report 2020
To find progress on health outcome indicators, visit the World health statistics





.tmb-1920v.jpg?sfvrsn=99ca5a94_1)
.tmb-1920v.jpg?sfvrsn=5aedd94d_1)