Interagency Emergency Health Kit 2024
IEHK 2024 is designed to serves an outreach population of 10’000 people for 3 months
The IEHK Secretariat called for a revision end of 2022 with the technical committee. The IEHK 2024, as with prior versions, has incorporated updates, including additions, deletions and changes. These are based on newer treatments that have become available and also on the increasing complexity emergency contexts.
The new IEHK 2024 undergo a redefinition of the subcomponents: the IEHK 2024 is divided into two modules : basic and supplementary, and those modules are then subdivided into units. A "unit" refers to product categories within a module. Each unit is packaged based on its product category to facilitate proper distribution upon reaching its destination.
The procurement is done at unit level, each unit is standalone than can be requested in different quantities depending on the needs.
Structure of the new IEHK 2024
The Basic module: This module contains products appropriate for use, services and staffing at Primary Health Care levels, or outpatient services that may be available at higher level facilities. It includes oral medicines (only) and other supplies that correspond generally to the level and training of nurses and community health care workers. The quantification for one basic module is based on an outreach population of 1,000 people over an estimated period of 3 months.
Structural changes in the basic module
The " basic medicines and renewable unit" from previous editions have been combined into a single unit. This unit now includes both medicines and all items considered "renewable," simplifying the reordering process.
The "basic equipment unit" in this version includes only reusable items, kept as a separate unit due to its different reorder patterns. This approach prevents duplication of durable medical devices.
The Supplementary module: This module contains products appropriate for use, services and staffing at the hospital level. It includes medicines (orals and injectables) and other supplies that correspond generally to the level and training of physicians, nurses and other staff with specialized training. This module is designed for hospital use or larger health centres with specialized services. The outreach population for hospitals is generally larger, here 10’000 people and attendance is higher, also over an estimated period of 3 months.
Structural changes in the supplementary module
In addition to the nomenclature changes (kit/module/unit), additional medicines units were created in the supplementary module. In total, seven different types of supplementary medicines units were designed to simplify distribution and provide flexibility in ordering. The previous IEHK versions included 5 supplementary parts containing medicines. Four of these parts remain unchanged, while the main supplementary medicines part is now divided into three units as shown below.

The decision split the supplementary module into different of supplementary medicines units is based on two main factors. First, the new IEHK 2024 supplementary module contains more types of medicines, which presents several challenges. Logistically, it extends the lead time for assembling one module (from the first component to the last), which in turn impacts the shelf life of the products. It also increases the total weight and volume. Second, from a therapeutic standpoint, some medicines need to be handled by trained healthcare workers and are used in different hospital wards. Due to these factors, it was decided to divide the old 2017 supplementary medicine part into three different units.
- Supplementary medicines oral unit: for general hospital wards and can also be directed/ repurposed to hospital OPDs, mobile clinics, or large health centres;
- Supplementary medicines injectable unit: injectable medicines for general hospital wards.
- Supplementary medicines metal health unit : psychotropics for use by trained professional in general hospital wards or specialized hospitals but which require special handling.
The cold chain, PEP, malaria and controlled unit remain. Note that the supplementary malaria unit is not labelled as "medicine" because it includes not only medicines but also all the materials required to reconstitute and administer artesunate 60 mg/ml.
Below design represents the different units and their intended level of care.
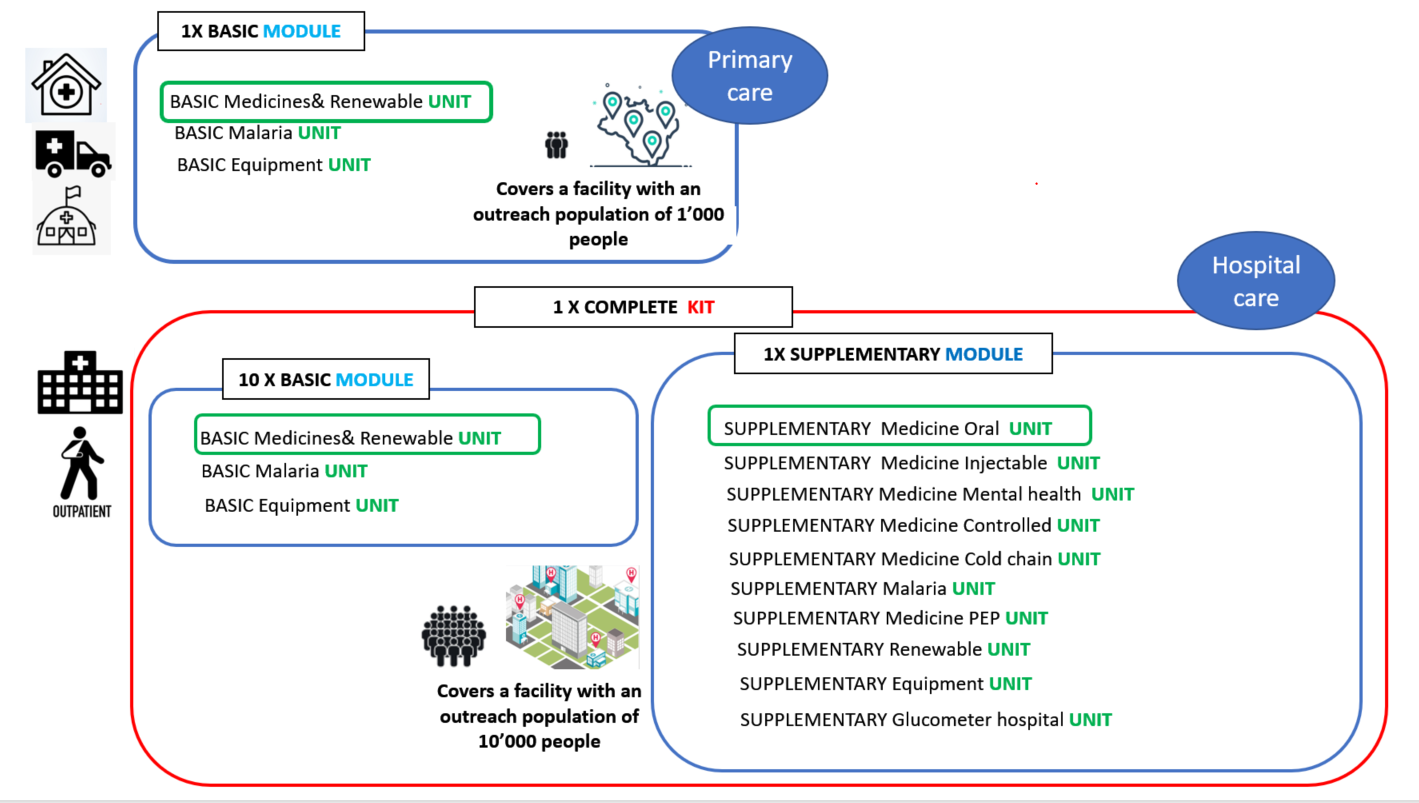
Composition of the IEHK 2024

Please note that for WHO projects, the mental health kit (MHK 2022) should be requested instead of the mental health unit to ensure a more comprehensive response.
Major changes in the IEHK 2024
To get an overview of the major structural and content changes in the kit, please refer to the booklet. It lists all the changes made by module/unit.
Procurement
- WHO programmes and Regional Offices, Country Programmes, UNAIDS: the IEHK 2024 can be ordered using a catalogue GSM requisition
- Non-WHO entities: please contact WHO Procurement Services at procurement@who.int, stating” IEHK 2024” in the subject of the message. WHO Procurement Services will provide guidance for a direct procurement
History of the Interagency Emergency Health KitThe Interagency Emergency Health Kit (IEHK) was first developed in 1990. Since its inception it has been...
Previous version IEHK 2017- transition to the new IEHK 2024
The update of the IEHK took more than a year, in addition to the time required for the contractual process and for suppliers to assemble the new IEHK 2024. The first units will be ready for purchase by the end of 2024 or the beginning of 2025, depending on the agency assembling the kit. The end of 2024 will serve as a transition period for the IEHK, during which both versions might be available in the field.
Unless there are significant changes in international treatment guidelines, the IEHK 2024 is expected to remain in use until 2030, date set for the next revision.


