
Standard Material Transfer Agreements (SMTA2) and other contracts
What is an SMTA2?
The SMTA2 is one of the two PIP Framework Benefit Sharing mechanisms. It is a legally-binding contract between WHO and an influenza product manufacturer, research institution, or other entity that receives PIP Biological Materials (PIP BM), such as influenza viruses with pandemic potential (IVPP), from a laboratory which is part of the Global Influenza Surveillance and Response System (GISRS). In exchange for access to PIP BM, the entity commits to provide to WHO, benefits that can be used to prepare for (e.g. training, technology license) or respond to (e.g. vaccines, antivirals, diagnostic kits) pandemic influenza. By concluding SMTA2s and other contracts now, WHO will have predictable access to pandemic response products, such as vaccines, antivirals and diagnostics, when they are most needed.
Who is required to sign an SMTA2?
The model SMTA2 set out in Annex 2 of the PIP Framework was amended by the 72nd World Health Assembly. The text of the amendment and a set of Q&As are available below.
How are recipients of PIP BM traced and notified?
Transfers of PIP BM - including IVPP - are tracked through the Influenza Virus Traceability Mechanism (IVTM). The IVTM is a publicly accessible, online system that records the transfer and movement of PIP BM into,
within and to parties outside GISRS.
Signed SMTA2s and benefits secured
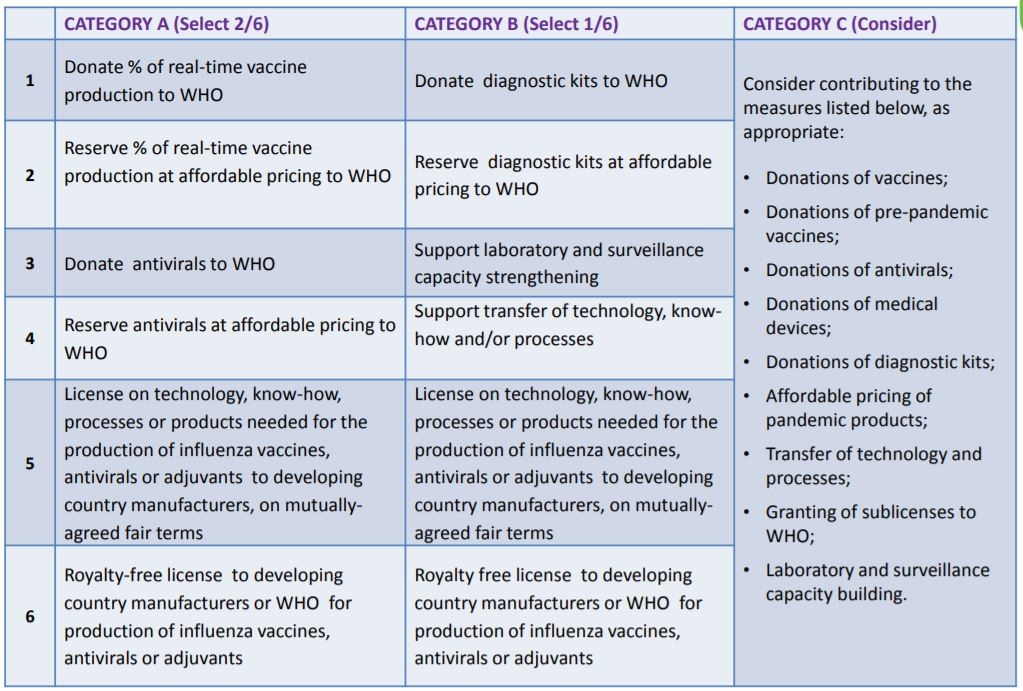
The purpose of the SMTA2 is to obtain legally binding commitments from users of PIP BM (including IVPP) to share certain benefits with WHO. Recognizing that there are different types of recipients PIP BM, the signatories are classified into the following three different categories:
- Category A: Influenza vaccine and antiviral manufacturers
- Category B: Manufacturers of other pandemic related products (e.g. diagnostic kits)
- Category C: All other recipients (universities, research institutions, bio-tech companies, etc.)
The recipients, depending on their nature and capacity, choose from the specific list of benefit options found in Annex 2, Article 4 of the PIP Framework and summarized in the table.
Manufacturers of vaccines and/or antivirals (Category A)
To date WHO has concluded 15 agreements with vaccine and antiviral manufacturers and through these SMTA2s has secured approximately four times the amount of pandemic vaccine available during the H1N1 pandemic. These signatories include 5 multinational companies and the largest developing country manufacturer.
Manufacturers of other pandemic related products (Category B)
Under this category WHO is securing access to products such as diagnostic test kits, ancillary products as well as resources to conduct training. To date, agreements have been signed with 2 large diagnostic manufacturers, providing WHO with a supply of diagnostic kits and syringes during the next pandemic.
Academic & research institutions (Category C)
To date WHO has concluded 76 agreements with academic and research institutions and, although not required, 29 have offered to provide a benefit-sharing contribution. Most of these institutions have offered to provide Laboratory and Surveillance Capacity Building (training) as their contribution.
Other Agreements
In addition to SMTA2s, the PIP Framework enables WHO to enter into other agreements to promote pandemic influenza preparedness and response, including access to pandemic and inter-pandemic influenza products.
SMTA2 4-year review process
In accordance with article 6.2 of the SMTA2, all the agreements with manufacturers (Categories A and B) are to be reviewed at a minimum every 4 years. The purpose of this process is to ensure that the SMTA2s are up to date and ready to be operationalized in the event of an influenza pandemic. The 4-year review is intended to assess whether any material circumstances or processes have changed for the company or WHO, requiring changes to the documents. When this is the case, the parties renegotiate as necessary. If a new agreement is signed, it replaces the previous agreement which becomes null and void. This website only contains currently applicable SMTA2s.
WHO Training on Laboratory Quality Management and Biosafety for National Influenza Centres
WHO collaborated with the University of Siena to host a training workshop on Laboratory Quality Management and Biosafety for National Influenza Centres (NICs). This training - originating through the SMTA2 process under the PIP Framework - was the first of its kind organized by the WHO Influenza Preparedness and Response division (IPR). By strengthening global laboratory surveillance capacity, it supports the achievement of the PIP Framework goal of improving pandemic preparedness and response.
The training was tailored towards NICs in-need of support, identified based on the 2017 NIC performance review process conducted by the Global Influenza Programme. NICs from each of the 6 WHO regions were selected and 13 participants – quality/biosafety officers and senior lab technologists - from the following 12 countries attended the workshop:
- AFRO: Liberia, Mauritius
- AMRO: Argentina, Trinidad and Tobago
- EMRO: Kuwait, Lebanon, Sudan
- EURO: Czechia
- SEARO: Indonesia, Myanmar
- WPRO: Malaysia, Viet Nam

For further information about the training activities, please see the training workshop report and the agenda below.

