This Medical Product Alert relates to falsified Verorab® vaccines that have been identified in the Philippines and reported to WHO. This vaccine is used for the prevention of rabies in children and adults. It can be used to protect those who are at risk of exposure to rabies (pre-exposure vaccination) or to prevent the development of rabies after exposure has occurred, usually following the bite of an animal suspected of having rabies (post-exposure prophylaxis). Its genuine version is manufactured by Sanofi Pasteur.
Rabies is a vaccine-preventable viral disease that is almost always fatal following the onset of clinical symptoms. Rabies is present on all continents, with over 95% of human deaths occurring in the Asia and Africa regions.
In December 2018, the Philippines Food and Drug Administration (FDA) issued a public health warning concerning falsified Verorab® vaccines circulating in the country. Since that date, a further batch of falsified Verorab® vaccines has been reported. Investigations are ongoing and laboratory analysis is underway to better assess the potential risk to public health.
Two falsified vaccines have so far been discovered and their details and available photographs are shown below.
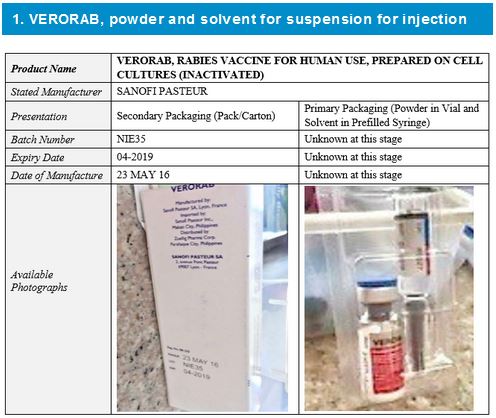
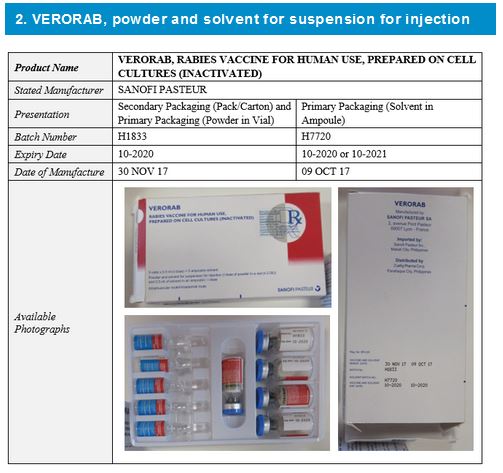
It should be noted that:
- The stated manufacturer, Sanofi Pasteur, has confirmed they did not manufacture these falsified vaccines.
- Variable data shown in this alert do not correspond to the genuine manufacturer records.
- There have been no known adverse reactions reported to WHO at this stage.
WHO requests increased vigilance within the supply chains of countries likely to be affected by these falsified vaccines. Increased vigilance should include hospitals, clinics, health centres, wholesalers, distributors, pharmacies and any other suppliers of vaccines.
If you are in possession of the above vaccines, please do not use. If you have used these falsified vaccines, or if you suffer an adverse event having used these vaccines, please seek immediate advice from a qualified healthcare professional, and ensure they report the incident to your local Ministry of Health/National Medicines Regulatory Authorities/National Pharmacovigilance Centre.
All medical products must be obtained from authentic and reliable sources. Their authenticity and condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
National health authorities are asked to immediately notify WHO if these falsified vaccines are discovered in their country. If you have any information concerning the manufacture, distribution, or supply of these vaccines, please contact: rapidalert@who.int
Substandard and Falsified (SF) Medical ProductsAll WHO Drug Alerts are available at the following link:
Full List of WHO Medical Product Alerts
To sign up for WHO Medical Product Alerts, please visit:
https://covid.comesa.int/teams/regulation-prequalification/regulation-and-safety/rss
