This Medical Product Alert relates to confirmed falsified Augmentin (Amoxicillin trihydrate – Potassium clavulanate) found in Uganda and Kenya. It should be noted that this is the second WHO Medical Product Alert issued on falsified Augmentin in the African region. The first WHO Medical Product Alert N°2/2018 was issued on 2 March 2018.
Genuine amoxicillin + clavulanic acid is used to treat a range of bacterial infections and is listed on the WHO Essential Medicines List as an access group antibiotic.
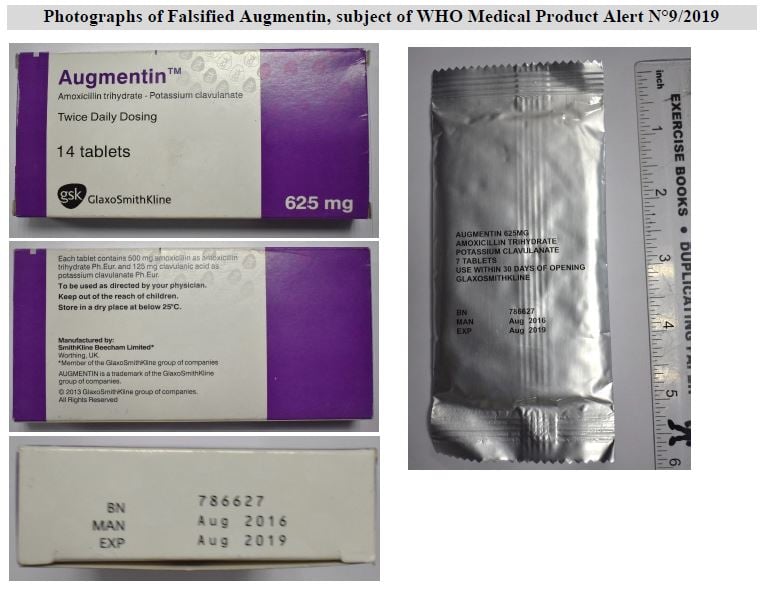
WHO was recently informed by the Uganda National Drug Authority that falsified Augmentin was found at patient level in Uganda having been discovered through routine post marketing surveillance on the quality of medical products in the market. Samples were sent for quality assurance laboratory testing and revealed none of the expected active ingredients. The Kenya Pharmacy and Poisons Board confirmed that the same batch of falsified Augmentin had previously been found at patient level in Kenya.
Product details are listed in Table 1 below and contained in the Uganda National Drug Authority News Release.

It should be noted that:
- The stated manufacturer has confirmed they did not manufacture this falsified version.
- Quality assurance laboratory analysis did not identify any of the expected active ingredients.
- At this stage, no adverse reactions have been reported to WHO.
- There are labelling and packaging inconsistencies.
Photographs and advice to the public are available below.
WHO requests increased vigilance within the supply chains of countries likely to be affected by this falsified medical product. Increased vigilance should include hospitals, clinics, health centres, wholesalers, distributors, pharmacies and any other suppliers of medical products.
If you are in possession of the above specific product, please do not use. If you have taken this falsified medical product, or if you suffer an adverse event or an unexpected lack of efficacy, please seek immediate advice from a qualified healthcare professional, and ensure they report the incident to your local Ministry of Health/National Medicines Regulatory Authorities/National Pharmacovigilance Centre.
All medical products must be obtained from authentic and reliable sources. Their authenticity and condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
National health authorities are asked to immediately notify WHO if this falsified medical product is discovered in their country. If you have any information concerning the manufacture, distribution, or supply of this medical product please contact rapidalert@who.int
WHO Global Surveillance and Monitoring Systemfor Substandard and Falsified Medical Products
For further information, please visit: http://covid.comesa.int/medicines/regulation/ssffc/en/
