This Medical Product Alert relates to the circulation of confirmed falsified Mencevax ACWY in the WHO region of Africa. Genuine Mencevax ACWY is used to prevent meningitis types A, C, W and Y.
On 15 March 2019, the Ministry of Public Health in Niger issued a call for vigilance regarding the circulation of falsified Mencevax ACWY vaccines. This WHO medical product alert provides the details of those products which have been confirmed as falsified to date. Inquiries indicate that the supply chain route of these falsified vaccines includes other countries in West Africa: as such, increased vigilance is requested in all countries of the region, at all levels of the supply chain.
A vaccination campaign against meningitis A targeting children from 1 to 7 years old is ongoing in Niger.
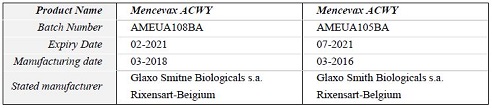
Table 1: Details of falsified Mencevax ACWY vaccines, subject of WHO Alert N°5/2019
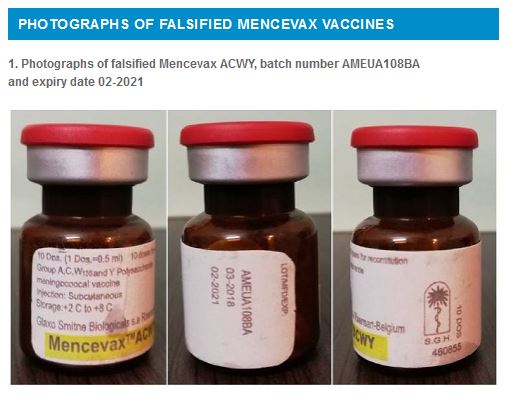
The language on the labels of the above falsified Mencevax ACWY vaccines display text in English language.
Samples are being sent for laboratory analysis to determine their contents and better assess the risk to public health. This medical product alert n°5/2019 will subsequently be updated and posted on the WHO website once laboratory results are known. No serious adverse reactions attributed to these falsified vaccines have been reported to WHO at this stage.
Genuine Mencevax ACWY vaccine is manufactured by GSK for Pfizer which has the market authorization rights. With regards to the products detailed in the above table 1, both companies have stated that:
- they did not manufacture the above products
- the batch number and expiry date combinations displayed do not correspond to genuine manufacturing records
Page 2 shows photographs of the falsified vaccines subject of this alert and page 3 provides advice for healthcare professionals and patients.

WHO requests increased vigilance within the supply chains of countries likely to be affected by these falsified vaccines. Increased vigilance should include hospitals, clinics, health centres, wholesalers, distributors, pharmacies and any other suppliers of vaccines and medical products.
If you are in possession of the above batches of this vaccine, please do not use. If you have used the above batches of this vaccine, or if you suffer an adverse event or an unexpected lack of efficacy, please seek immediate advice from a qualified healthcare professional, and ensure they report the incident to your local Ministry of Health/National Medicines Regulatory Authorities/National Pharmacovigilance Centre.
All vaccines and medical products must be obtained from authentic and reliable sources. Their authenticity and condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
National health authorities are asked to immediately notify WHO if these falsified vaccines are discovered in their country. If you have any information concerning the manufacture, distribution, or supply of these products, please contact rapidalert@who.int
WHO Global Surveillance and Monitoring Systemfor Substandard and Falsified Medical Products
For further information, please visit: http://covid.comesa.int/medicines/regulation/ssffc/en/
