
WHO Coordinated Scientific Advice for health product R&D
WHO has developed the WHO Coordinated Scientific Advice procedure (CSA) whereby product developers may approach WHO and obtain advice on the most appropriate way to generate robust evidence on a product's benefits and risks with the view to obtain a WHO policy recommendation or obtain a prequalified status if the product is found to meet WHO requirements.

Coordinated scientific advice for health product R&D
The context
A key WHO mission is to enable access to high quality, safe and efficacious health products (diagnostics, medicines, vaccines, vector control products, medical devices) that are suitable for use in populations in greatest need. Two essential elements to achieve this goal are the generation of policy recommendations for health interventions and the WHO prequalification (PQ) assessment that rely on evidence from well-designed and well-conducted trials with appropriate endpoints to demonstrate their public health value as well as efficacy/performance and safety of the products. For this, the CSA procedure represents a novel standardized WHO corporate platform for interaction between product developers and the relevant technical department(s) and the Prequalification team within WHO.
What are the CSA Objectives?
The goal of the CSA procedure is to provide advice to product developers on the generation of robust data for future evaluation towards a WHO policy recommendation and product prequalification in areas of unmet public health needs.
The CSA Objectives
1. To provide advice to developers seeking to develop a health product in line with WHO’s expressed unmet public health needs in a given disease/condition area;
2. To ensure good understanding of WHO policy and PQ data needs and processes by developers as well as to provide feedback on specific areas of content for manufacturer’s development plans;
3. To accelerate timelines from clinical trial start to provision of data that would satisfy both policy and PQ requirements (depending on quality, safety and efficacy/performance data generated as well as any other relevant factors) and improve the quality of submissions received for PQ assessment and available for guideline development.
How does CSA work?
The CSA takes place based on a request from a product developer submitted to WHO. It follows an open submission process with a clearly defined single entry point that is the Science Division/Research For Health (SCI/RFH) department. WHO’s advice to the product developer will be coordinated between the relevant technical department(s) and the Prequalification Team through a formal and harmonized process.
It is expected that the product proposed by the developer are aligned with existing WHO Target Product Profiles (TPP) or Preferred Product Characteristics (PPC) that provide guidance on unmet public health needs. In the case where no such TPP/PPCs are available, the technical departments will indicate whether the submitted product aligns with a recognized unmet public heath need.
The CSA procedure applies to new products, or new/additional data on existing products with the view to assess their potential significant public health value.
Together, WHO experts from relevant Technical Department(s) and the Prequalification Team, with assistance from the Research for Health Department, will provide guidance to applicants on the suitability of the development plan and study designs for the generation of relevant data to ensure that the resulting evidence meets WHO’s standards for determining public health value.
WHO will provide Coordinated Scientific Advice on product development questions related to:
- quality
- non-clinical aspects
- clinical/epidemiological aspects of new products or updated forms of known products.
What is the CSA procedure?
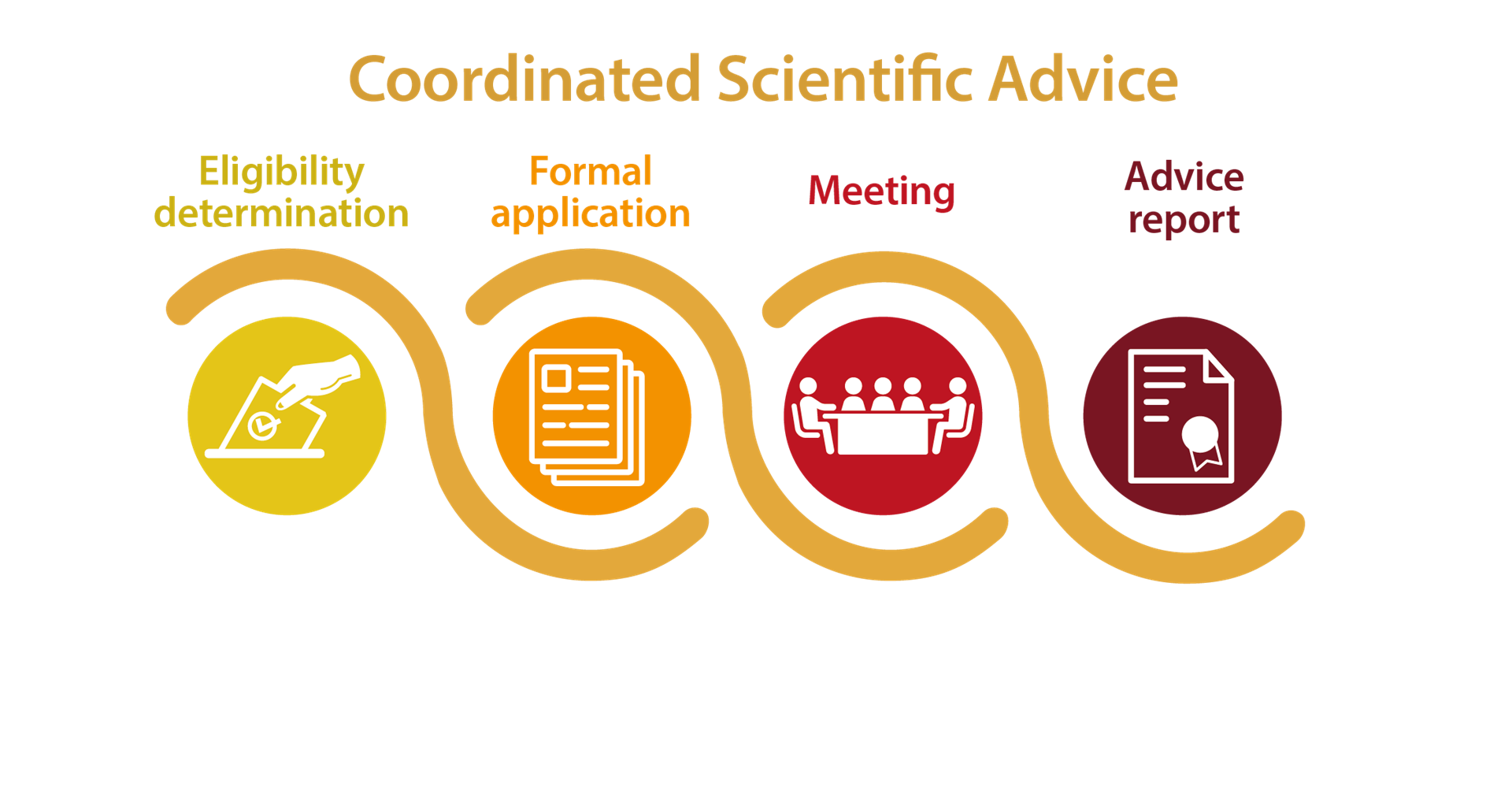
The CSA procedure takes place in 4 successive steps.
Step 1: Determination of Eligibility, where a request from the product developer is evaluated for eligibility, i.e. determines whether the suggested product meets pre-set eligibility criteria.
Step 2: Formal application, where the developer submits the full Submission Package to WHO for in-depth review by the technical department(s) and the Prequalification Team.
Step 3: Face-to-face/virtual meeting, during which the product developer gets an opportunity to meet the relevant WHO focal points and clarify any outstanding questions and address any issues related to the submission.
Step 4: Written report based on the review, outcomes and discussions held during the face meeting will be made available to the product developer.
Medicines, in vitro diagnostics, vaccines participate in this pilot phase.
Expected timeline for the CSA procedure
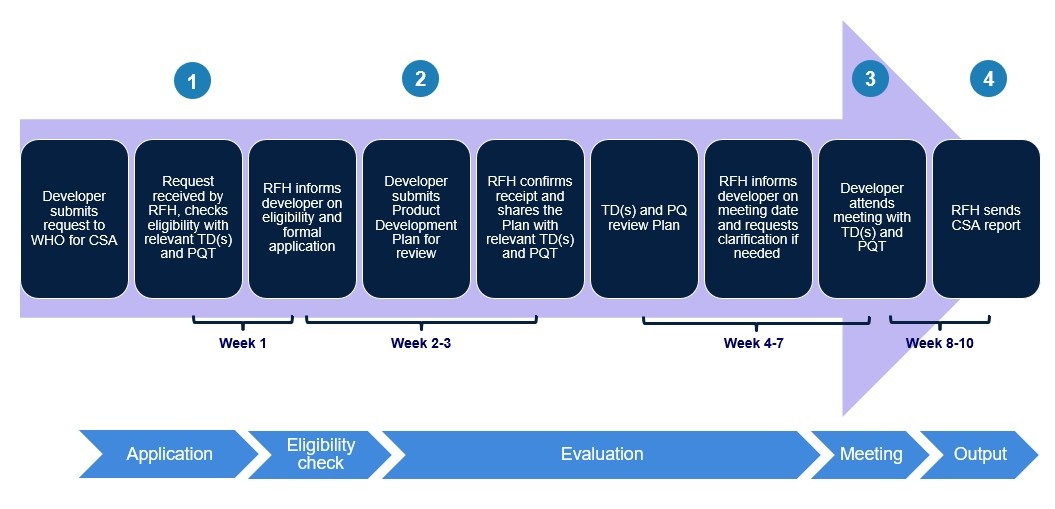
The timeline for the CSA procedure may vary depending on the submission received from the product developer, the nature of the product, its stage of development and the expected advice from WHO. However, the process is expected to take approximately ten weeks from the moment a request is received.
Before submitting a request for CSA
The optimal time for initiating CSA contact will vary for the different product streams but in general, it will be after clinical development has started and before the pivotal trial(s) design is/are finalized in the case of therapeutics
and vaccines, or before the start of clinical validation studies for IVDs. Under certain circumstances CSA would be considered at even earlier stages, such as at the discovery to development interface, as in the case of vector control products where
one of the driving forces for this process is the need to support innovation. Other circumstances may include when the novelty of the product class means that there are little to no established pathways for clinical evaluation. Ultimately, the timing
for CSA requests will be determined by the product developers’ needs based on the products under development.
Applicants should contact WHO before submitting a request for CSA. WHO is able to advise on all aspects of a submission, including format and contents. Evidently, a good-quality submission will streamline and accelerate the process.
For more information contact the RFH Department at: ScientificAdvice@who.int
Additional information:
Information for the completion of a Submission Package for the WHO Coordinated Scientific Advice procedure for health product R&D:
- Medicines (CSA_003)
- Vaccines (CSA_004, coming soon)
- In vitro diagnostics (CSA_005)
- Vector control products (CSA_006, coming soon)
Important to know
- Scientific advice will be on product development strategies; however, it is NOT a pre-evaluation of the product and will not itself confer any WHO endorsement.
- The scientific advice will not be binding on product developers and will not guarantee positive outcomes from PQ or policy recommendation, as those assessments will always be based on data not yet available at the time of the scientific advice.
Contact
Useful links
WHO is conducting a pilot of the CSA procedure. The findings and outcomes of the pilot will contribute to the finalization process and improvement of the CSA procedure.
Related activities in health R&D

