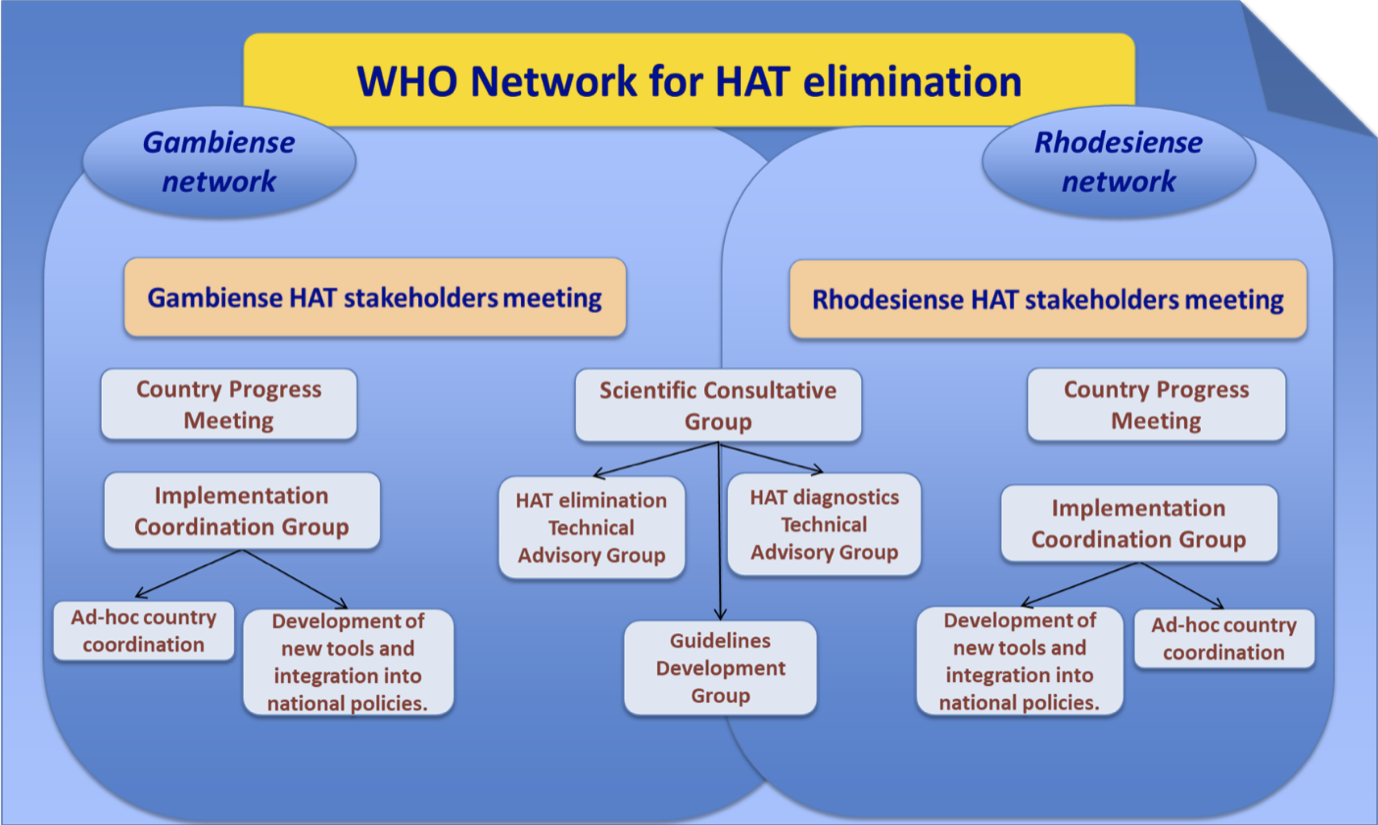
Partnership and WHO network for elimination
As we advance towards HAT elimination, coordination among all the partners in this endeavour is crucial. An essential role of WHO is to coordinate the efforts of the different partners with those of the national sleeping sickness control programmes (NSSCPs) in order to better structure the mechanisms of collaboration. To this end, WHO established the Network for HAT elimination to coordinate, strengthen and sustain efforts to eliminate the disease. The network links the NSSCPs and all the international organizations, research institutions, development agencies, nongovernmental organizations and donors involved in the control of HAT. The network was launched formally in March 2014 at the first HAT stakeholders meeting.
The HAT stakeholders meeting
The meeting of HAT stakeholders is the main forum for HAT elimination that brings together NSSCPs and the different stakeholders. It has been convoked every year since 2014 in Geneva, alternating gambiense-HAT and rhodesiense-HAT.
Meeting reports on gambiense-HAT
Report of the third WHO stakeholders meeting on gambiense human African trypanosomiasis elimination
Since 2000, concerted efforts by national programmes, supported by public–private partnerships, nongovernmental organizations, donors...
Report of the second WHO stakeholders meeting on gambiense human African trypanosomiasis elimination
In 2014, WHO convened the main stakeholders involved in the gambiense HAT elimination objective in order to strengthen the mechanisms of collaboration...
Report of the first WHO stakeholders meeting on gambiense human African trypanosomiasis elimination
In 2013, a WHO Expert Committee report was published, which provides a comprehensive update on new diagnostic tools, new therapeutic regimens and on the...

First WHO Meeting of Stakeholders on Elimination of Gambiense Human African Trypanosomiasis
The meeting reviewed the current epidemiological status of the disease and the recent achievements and challenges for moving towards the gambiense HAT...
Meeting reports on rhodesiense-HAT
Report of the second WHO stakeholders meeting on rhodesiense human African trypanosomiasis
The first WHO stakeholders meeting on r-HAT elimination (Geneva, 20–22 October 2014) was an important boost to the multisectoral coordination mechanism...
Report of the first WHO stakeholders meeting on rhodesiense human African trypanosomiasis
In April 2013, a WHO Expert Committee on human African trypanosomiasis control and surveillance updated the epidemiological patterns of the disease, diagnostic...

On the Road to Elimination of Rhodesiense Human African Trypanosomiasis: First WHO Meeting of Stakeholders
Human African trypanosomiasis (HAT), also known as sleeping sickness, has been a major scourge afflicting populations in Africa in areas where its specific...
The annual coordination and progress meeting of all endemic countries
The annual coordination and progress meeting is a key event that convenes focal points for HAT from the different endemic countries in order to define WHO support to NSSCPs after reviewing the situation and planning activities.
Annual meetings have been held in Yaoundé (March 2015); Geneva (March 2016); Conakry y 2017); Geneva (April 2018) and Grand Bassam (February 2019).
The Scientific Consultative Group
The Scientific Consultative Group includes advisory groups of acknowledged experts who consider identified knowledge needs and gaps within the context of the HAT elimination strategy. Currently it includes:
- the HAT elimination Technical Advisory Group (HAT-e-TAG), constituted in 2016 to advise on the HAT elimination process, including strategies for elimination, development of indicators, and criteria and procedures for assessing and monitoring HAT elimination. It meets in Geneva annually (November 2016, November 2017, November 2018, November 2019);
- the Guideline Development Group (GDG), convened in 2017 to update the WHO guidelines on HAT therapeutics (introduction of fexinidazole); and
- the HAT diagnostics technical advisory group (HAT-D-TAG), convened in 2020 to define the needs for HAT diagnostics and the priorities for HAT diagnostics research.
Other activities within implementation coordination network groups and subgroups
Between 2014 and 2020, the different groups and subgroups of the network conducted various activities.
- Ad-hoc country coordination meetings
Support to occasional national or regional meetings in coordinating the different stakeholders collaborating with the NSSCP of the respective countries.
Ad hoc country coordination meetings have been organized in Angola, Angola–Congo–Democratic Republic of the Congo (within the framework of transboundary collaboration to eliminate HAT supported by the Foundation for Innovative New Diagnostics, FIND); Benin–Togo (regular workshops in Cotonou and Lomé to assess elimination and plan surveillance in historical foci); Chad (regular trypanosomiasis and tsetse partners' harmonization meetings); Côte d’Ivoire (meetings within the framework of the research and control network on trypanosomiasis and tsetse organized by the French National Institute for Research and Development (IRD) and the national HAT elimination programme, PNETHA); Democratic Republic of the Congo (frequent meetings twice or three times a year) partner meetings led by the national HAT control programme (PNLTHA) with the support of WHO and the Institute of Tropical Medicine in Antwerp, ITM), Guinea; South Sudan; Uganda (co-organized with the Coordinating Office for Control of Trypanosomiasis in Uganda, COCTU), Uganda–South Sudan (within the transboundary collaboration to control HAT supported by FIND).
- Development of new tools and Integration in national policies Group
The Group provides a forum to discuss specific subjects about the development and implementation of new tools with the partners involved and their integration in national policies. Different subgroups have been convoked to work in new tools for diagnostics and treatment:
- update of the methodological framework for HAT clinical trials (meeting in September 2014);
- first meeting, to agree next steps after the phase III clinical trial of the new oral drug fexinidazole (December 2014);
- second meeting, to advance HAT diagnostics (May 2015);
- third meeting, to follow up on new oral drugs for HAT (June 2015);
- fourth meeting, to follow up on new oral drugs for HAT (December 2015);
- fifth meeting, to advance HAT diagnostics (October 2016);
- sixth meeting, to review oral treatment for rhodesiense HAT and perspectives for a trial of fexinidazole (December 2016);
- seventh meeting, to review the fexinidazole access plan for gambiense HAT (December 2016);
- eighth meeting, to update the fexinidazole access plan for gambiense HAT (October 2017);
- ninth meeting, to update the access plan for new oral drugs including acoziborole (January 2019); and
- tenth meeting, to explore the possible extended use of a single-dose oral drug for gambiense HAT (December 2019).
The network has conducted multiple activities involving the different technical groups described and NSSCP partners involved in HAT elimination. WHO will continue to follow this strategy of collaborative discussion and development of new strategies to overcome the anticipated obstacles. Two new thematic subgroups have been proposed and specific meetings are planned: vector control and socio-anthropology. These subgroups could bring new perspectives to the global understanding of HAT and, consequently, make a very positive contribution towards its elimination.
Collaborating centres
Currently, four scientific institutions have been identified as WHO collaborating centres to provide technical support to WHO’s programme:
- the Parasite Diagnostics Unit of ITM in Antwerp (Belgium), as WHO Collaborating Centre for Research and Training in human African trypanosomiasis diagnostics;
- the Research Unit of IRD, based in Montpellier (France), with antennas in the International Centre for Research and Development in Livestock in Sub Humid Areas (CIRDES) in Bobo Dioulasso (Burkina Faso) and the Institut Pierre Richet (IPR) in Bouake (Côte d’Ivoire) as WHO Collaborating Centre for research in host–vector–parasite interactions to sustain surveillance, control and elimination of human African trypanosomiasis;
- the Institut national de recherche biomédical (INRB) in Kinshasa (Democratic Republic of the Congo), as WHO Collaborating Centre for reference and training in diagnosis of human African trypanosomiasis; and
- the Clinical Investigation and Access to BioResources Platform (ICAReB) at the Institut Pasteur in Paris (France) as WHO Collaborating Centre for the human African trypanosomiasis Biobank.
Public–private partnerships
Sanofi
The pharmaceutical firm Sanofi is the result of a merger between Aventis Pharma and Sanofi. As a result of joint international efforts and public awareness, an agreement was established with Aventis Pharma in May 2001 for a 5-year contribution to fight sleeping sickness, including the donation of three specific drugs for treatment of sleeping sickness and financial support to strengthen control and surveillance at national level. This collaborative agreement was successively renewed in 2006 (and expanded to include several neglected tropical diseases), in 2011 and in 2016.
Bayer
The pharmaceutical firm Bayer produces two drugs of major importance to HAT treatment: suramin and nifurtimox. The latter is used off-label in combination with eflornithine. In 2002, Bayer agreed to donate suramin to WHO for the treatment of first‐stage rhodesiense sleeping sickness patients and in 2009 expanded its donation to nifurtimox for its use in combination with eflornithine in the treatment of second‐stage gambiense HAT.
Other established partnerships
The Drugs for Neglected Diseases initiative
The Drugs for Neglected Diseases initiative (DNDi), established in 2003, is a collaborative, non-profit drug research and development (R&D) organization that is developing new treatments for neglected diseases. DNDi aims to deliver new oral treatments to cure sleeping sickness that are safe, affordable, effective and easy to use, and support the sustainable elimination of the disease. DNDi works in the following areas for HAT:
- research for better use of existing medicines (e.g. NECT); and
- identifying, developing and delivering new medicines (e.g. fexinidazole, the first all-oral treatment for gambiense HAT, and acoziborole, the first single-dose oral treatment for gambiense HAT. DNDi is now evaluating the safety and efficacy of fexinidazole to treat rhodesiense HAT.
The Foundation for Innovative New Diagnostics
The Foundation for Innovative New Diagnostics (FIND), an initiative for the development of new diagnostic tests for poverty related-diseases, was launched in May 2003. FIND and WHO collaborate in the development of the urgently needed diagnostic tests to support the elimination of sleeping sickness. FIND works in the following areas for HAT:
- rapid diagnostic tests (RDTs) for gambiense HAT, using native and recombinant antigens;
- RDTs for gambiense HAT combined with malaria;
- loop-mediated isothermal amplification, or LAMP;
- improvements in parasitological methods; and
- strategies for strengthening passive screening.
The Programme Against African Trypanosomiasis
The Programme Against African Trypanosomiasis (PAAT) was established in November 1997 to provide overall direction and focus to the activities of all those concerned with and affected by this disease. The programme has been based on the collaboration and coordination between United Nations agencies, international organizations, research institutes, donors, development agencies, governments and affected rural communities. Programme management is delegated to a joint secretariat composed of FAO, AU/IBAR, IAEA and WHO. The goal of the programme is to identify a solution to the problem of tsetse and trypanosomiasis, both human and animal, in the broader context of food security, health, rural development and sustainable agriculture.
The Pan African Tsetse and Trypanosomiasis Eradication Campaign
The Pan African Tsetse and Trypanosomiasis Eradication Campaign (PATTEC) was created following a decision by the African Heads of State and Government at the African Union Summit in Lomé, Togo in July 2000 (Decision AGH/Dec.156-XXXVI). The Secretary General of the African Union was charged with the task of initiating a campaign to eradicate the trypanosomiasis and tsetse fly menace from the African continent, once and for all.
The African Union Inter-African Bureau for Animal Resources
The African Union Inter-African Bureau for Animal Resources (AU-IBAR), a specialized technical office of the African Union, was established in 1951. Initially known as the Inter-African Bureau of Epizootic Diseases and mainly concerned with rinderpest control, its mandate was expanded to other major animal diseases in 1956 and finally to all aspects of animal resource development in 1970. Trypanosomiasis, which is considered a major obstacle to African agricultural development, is of particular concern to AU‐IBAR.
AU-IBAR is the secretariat of the International Scientific Council for Trypanosomiasis Research and Control (ISCTRC). The ISCTRC was established in the 1960s as a vehicle of cooperation and implementation across national, regional and continental barriers. ISCTRC is a statutory council of the African Union that acts as a platform for knowledge sharing and information dissemination on trypanosomiasis research and control. It organizes a biennial scientific conference to evaluate progress sustained since 1949.
Many other institutions in academia, research institutions, NGOs, foundations, bilateral cooperation organisms also collaborate in the control and surveillance of HAT.