
2.4 Drug-resistant TB: treatment enrolment, coverage and outcomes
People with drug-resistant TB (DR-TB) need treatment with regimens that include second-line drugs, such as bedaquiline and fluoroquinolones (1). WHO recommends different regimens for rifampicin-resistant TB (RR-TB) or multidrug-resistant TB (MDR-TB, defined as resistance to both rifampicin and isoniazid); isoniazid-resistant TB; pre-extensively drug-resistant TB (pre-XDR-TB, defined as TB that is resistant to rifampicin and any fluoroquinolone) and XDR-TB (resistance to rifampicin, any fluoroquinolone and at least one of bedaquiline or linezolid). These regimens are more expensive (US$ 400–600 based on prices quoted by the Global TB Drug Facility) compared with first-line treatments for drug-susceptible TB (around US$ 50 per person) (2). They also cause more adverse events.
Since 2018, WHO has recommended all-oral regimens for the treatment of MDR/RR-TB, a landmark advance compared with previous regimens that included injectable agents (1). The latest recommendations for treatment of drug-resistant TB include three major categories of regimen (3,4). The first category consists of two 6-month all-oral regimens for people with MDR/RR-TB (with or without resistance to fluoroquinolones). The second category includes several all-oral short regimens of 9 months for people with MDR/RR-TB who do not have any resistance to fluoroquinolones. The third category includes longer regimens of 18–20 months that may include an injectable drug (amikacin). The 6-month regimens are prioritized for use while the longest regimens are a last resort.
Globally in 2023, 175 923 people were enrolled on treatment for MDR/RR-TB. This was a small decrease from 177 912 in 2022 (-1.1%). Most of those enrolled on treatment were people aged ≥15 years (Fig. 2.4.1).
Trends in the numbers of people newly diagnosed with RR-TB or MDR-TB (MDR/RR-TB) and enrolled on treatment for MDR/RR-TB between 2010 and 2023 vary considerably among the 30 high MDR/RR-TB burden countries (Fig. 2.4.2). In general, treatment enrolments are close to the numbers of people newly diagnosed.
The numbers of people being detected with MDR/RR-TB and enrolled on treatment fall far short of the estimated number of people developing MDR/RR-TB (incident cases) each year (Fig. 2.4.3). Closing the gap requires improvements in overall detection of people with TB (Section 2.3), improvements in the percentage of those diagnosed with TB who are bacteriologically confirmed (necessary to test for drug resistance) and improvements in the coverage of testing for RR-TB (Section 2.2).
Estimated levels of treatment coverage for MDR/RR-TB, approximated as the number of people enrolled on treatment for MDR-TB in a given year divided by the estimated number of people who developed MDR/RR-TB (incident cases) in the same year and expressed as a percentage, remain low: globally, 44% in 2023 (Fig. 2.4.4). The WHO region with the highest level of treatment coverage in 2023 was the European Region.
Among the 30 high MDR/RR-TB burden countries, only nine had treatment coverage levels of ≥50% in 2023: Azerbaijan, Belarus, India, Kazakhstan, Peru, the Republic of Moldova, the Russian Federation, South Africa and Uzbekistan. Those with particularly low levels (<20%) included the Democratic People’s Republic of Korea, the Democratic Republic of the Congo, Myanmar, Nepal and Papua New Guinea (Fig. 2.4.5).
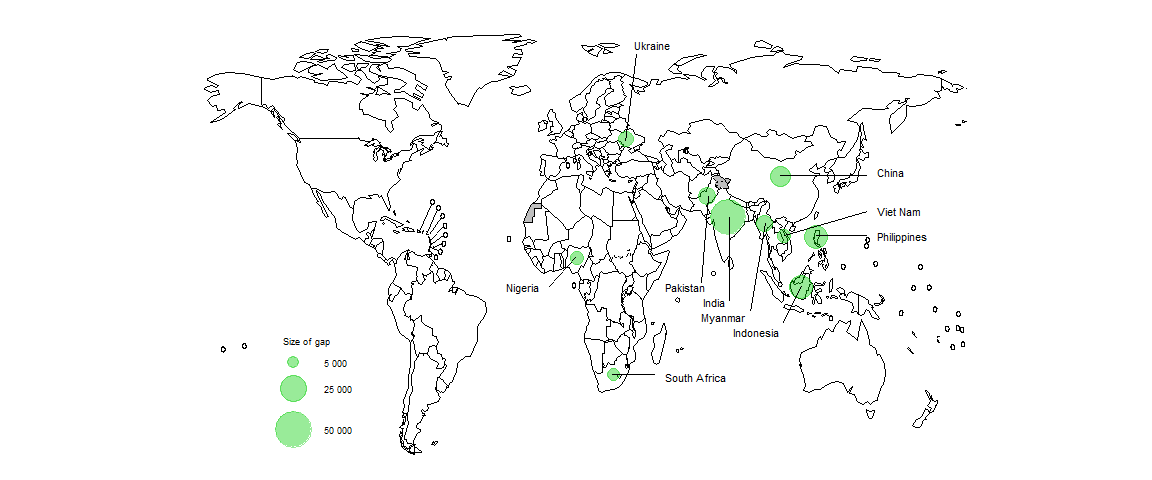
In 2023, 10 countries accounted for 69% of the global gap between the estimated number of people who developed MDR/RR-TB and the number of people enrolled on treatment for MDR/RR-TB (Fig. 2.4.6).
Globally, the treatment success rate for people enrolled on treatment for MDR/RR-TB in 2021 (the latest year for which outcome data are available) was 68%; this was a further improvement from 64% in 2020, 60% in 2019 and much better than the level of 50% in 2012 (Fig. 2.4.7).
Among WHO regions, the treatment success rate in 2021 was lowest in the European Region and highest in the Eastern Mediterranean Region (Fig. 2.4.8).
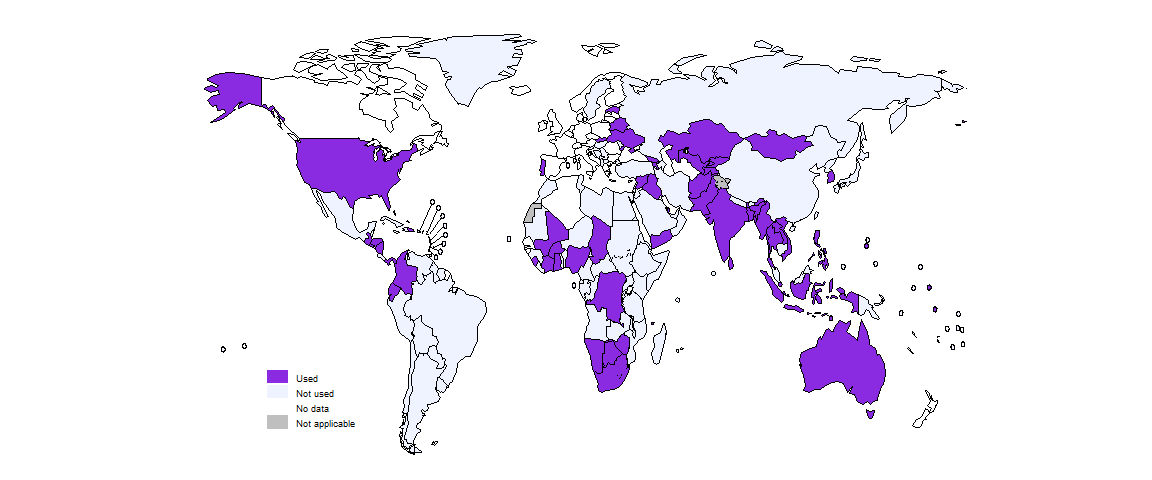
Following its approval by WHO in 2022, the short 6-month all-oral regimen known as BPaLM/BPaL is being used in a growing number of countries: 58 countries by the end of 2023, up from 41 at the end of 2022 (Fig. 2.4.9). Globally in 2023, 5646 people with MDR/RR-TB were reported to have been started treatment on the BPaLM/BPaL regimen, up from 1744 in 2022.
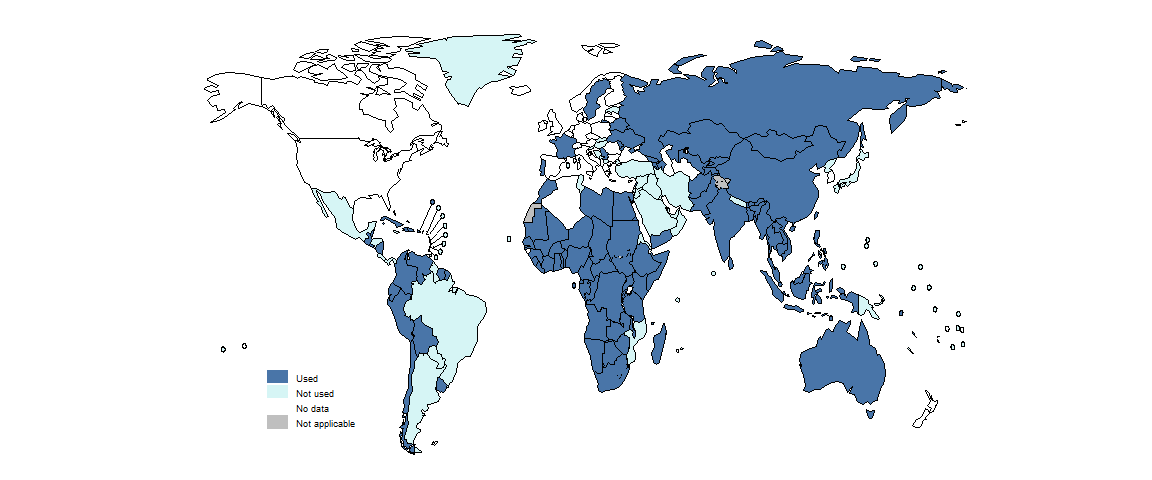
By the end of 2023, 100 countries were using the shorter 9-month regimens for the treatment of MDR/RR-TB (Fig. 2.4.10). This was a small increase from 95 in 2022 and 93 in 2021.
Globally in 2023, longer regimens (18–20 months) were still the most commonly used of three major categories of regimen for treatment of MDR/RR-TB; 41% of people with MDR/RR-TB were enrolled on treatment with longer regimens, followed by the all-oral 9-month (30%) and all-oral 6-month (3.2%) regimens (Fig. 2.4.11).
At regional level, the percentage of people with MDR/RR-TB for whom the all-oral 6-month regimen (BPaLM/BPaL) was used was highest in the African Region (7.8%) and the Eastern Mediterranean Region (9.4%). All-oral 9-month regimens were predominantly used in the African Region (61%).
Trends in the percentage of people with MDR/RR-TB who were treated with the three major categories of MDR/RR-TB regimens vary considerably among the 30 high MDR/RR-TB burden countries (Fig. 2.4.12). In 2023, seven countries used the all-oral 6-months regimen for at least 10% of people with MDR/RR-TB: Belarus, Myanmar, Nigeria, Pakistan, South Africa, Ukraine and Uzbekistan. More than half of those with MDR/RR-TB were treated with all-oral 9-month regimens in eight countries: Angola, Bangladesh, the Democratic Republic of the Congo, Nigeria, the Philippines, Somalia, South Africa and Viet Nam.
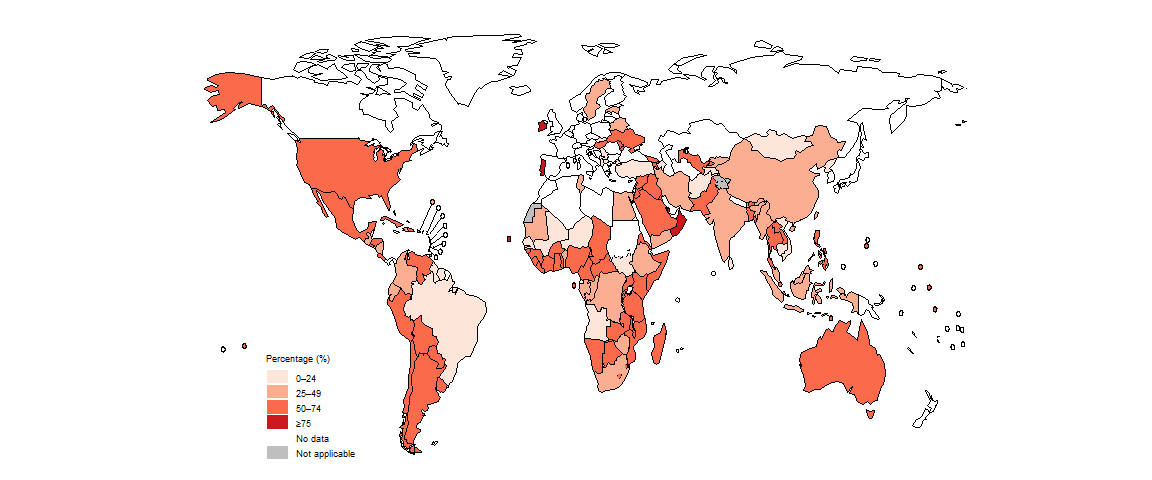
In 2023, in most countries, at least some people diagnosed with drug-resistant TB were being actively and systematically monitored for adverse events (Fig. 2.4.13).
Further country-specific details about treatment for drug-resistant TB are available in the Global tuberculosis report app and country profiles.
Data shown on this webpage are as of 29 July 2024 (see Annex 2 of the core report document for more details).
References
Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). Geneva: World Health Organization; 2018 (https://iris.who.int/handle/10665/275383).
Global Drug Facility (GDF). Geneva: Stop TB Partnership; 2024 (https://www.stoptb.org/buyers/plan-order).
WHO consolidated guidelines on tuberculosis, Module 4: Treatment – drug-resistant tuberculosis treatment, 2022 update. Geneva: World Health Organization; 2022 (https://iris.who.int/handle/10665/365308).
Key updates to the treatment of drug-resistant tuberculosis: rapid communication, August 2024. Geneva: World Health Organization; 2024 (https://iris.who.int/handle/10665/378472).
