This Medical Product Alert relates to 3 different falsified rabies vaccines (Verorab, Speeda, and Rabipur) and 1 falsified anti-rabies serum (Equirab) circulating in the Philippines. It is linked to the WHO Medical Product Alert N°1/2019 issued on 30 January 2019 regarding falsified Verorab rabies vaccines circulating in the Philippines. Rabies is a vaccine-preventable viral disease that is almost always fatal following the onset of clinical symptoms. Rabies is present worldwide, with over 95% of human deaths occurring in the Asia and Africa regions. Genuine Verorab, Speeda and Rabipur vaccines are used for pre-exposure vaccination or post-exposure prophylaxis. Genuine Equirab anti-rabies serum provides passive immunization against rabies.
WHO recently received confirmation that falsified batches of Verorab, Speeda, Rabipur and Equirab were available at patient level in the Philippines. Investigations are ongoing, and laboratory analyses are being facilitated for available samples to determine their contents and better assess the risk to public health. At this stage, no adverse reactions attributed to the below mentioned falsified products have been reported to WHO. A rabies vaccine shortage is ongoing in the Philippines.
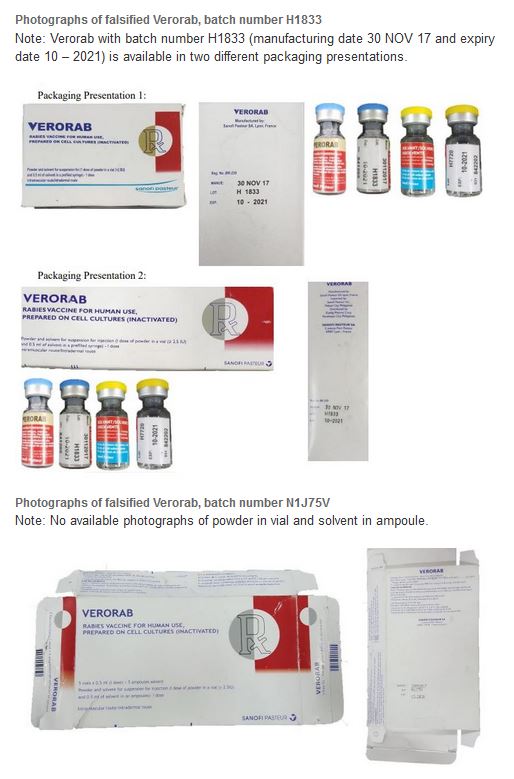
1. VERORAB, Rabies Vaccine for Human Use, Prepared on Cell Cultures (Inactivated)
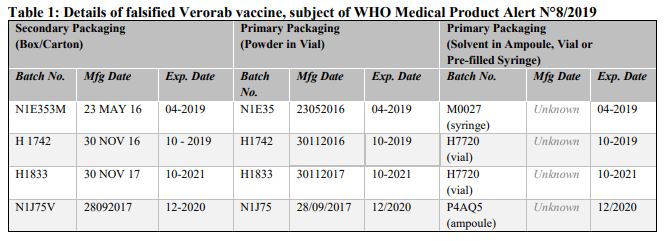
Falsified versions of 4 different combinations of batch numbers have so far been discovered. Product details are listed in Table 1 below and are also contained in the Philippines Food and Drug Administration Advisory No. 2019-190. Please refer to Annex 1 for available photographs.
Sanofi Pasteur, the genuine manufacturer and marketing authorization holder of Verorab, Rabies Vaccine for Human Use, Prepared on Cell Cultures (Inactivated) in the Philippines, stated:
- They did not manufacture the above listed products.
- The above listed products display labelling and packaging inconsistencies.
- The variable data do not correspond to the genuine manufacturing records.
- H1833, H1742, and N1J75V are falsified batch numbers.
- Batch number N1E353M is not a valid batch number for the Philippines market.
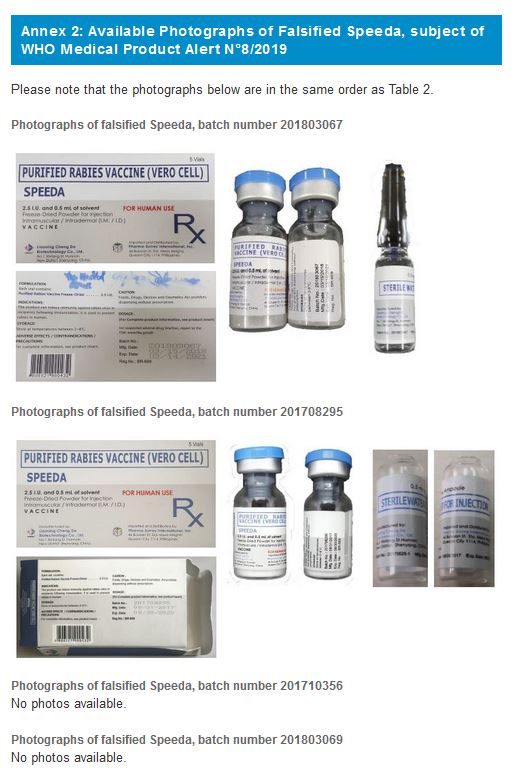
2. SPEEDA, Purified Rabies Vaccines (Vero Cell)
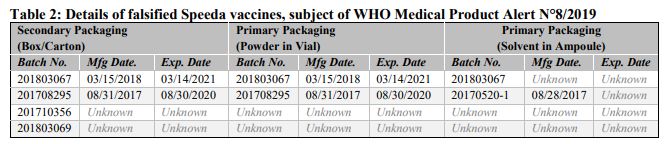
Falsified versions of 4 different combinations of batch numbers have so far been discovered. Product details are listed in Table 2 below and are also contained in the Philippines Food and Drug Administration Advisory No. 2019-153 Please refer to Annex 2 for available photographs.
Liaoning Cheng Da Biotechnology Co., Ltd., the genuine manufacturer of Speeda, Purified Rabies Vaccines (Vero Cell), stated:
- They did not manufacture the above listed products.
- The above listed products display labelling and packaging inconsistencies.
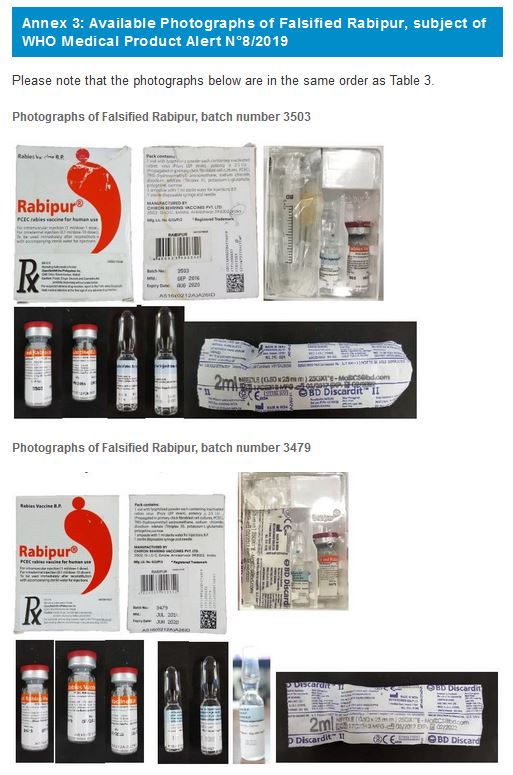
3. RABIPUR, PCEC rabies vaccine for human use
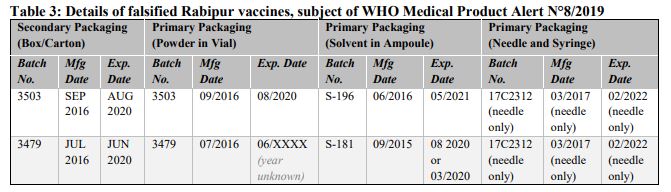
Falsified versions of 2 different combinations of batch numbers have so far been discovered. Product details are listed in Table 3 below and are also contained in the Philippines Food and Drug Administration Advisory No. 2019-170. Please refer to Annex 3 for available photographs.
GlaxoSmithKline (GSK), the genuine marketing authorization holder of Rabipur, and Chiron Behring Vaccines Pvt. Ltd, the genuine manufacturer of Rabipur, stated:
- They did not manufacture the above listed products.
- The above listed products display labelling and packaging inconsistencies.
- Since July 2017, Chiron Behring Vaccines Pvt. Ltd has not exported this product nor has GSK imported this product into the Philippines.
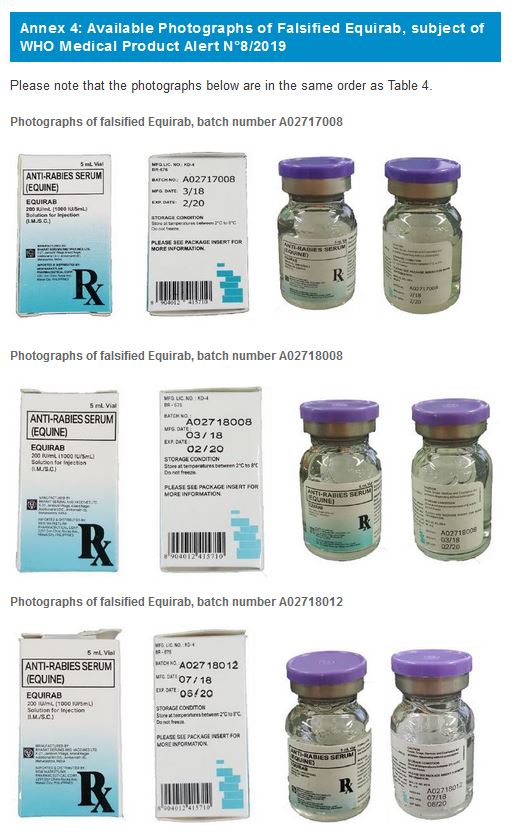
4. EQUIRAB, Anti-Rabies Serum (Equine)
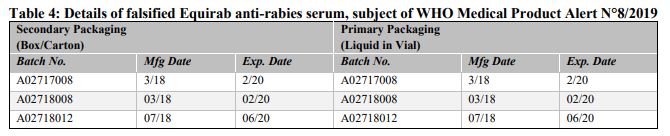
Falsified versions of 3 different combinations of batch numbers have so far been discovered. Product details are listed in Table 4 below and are also contained in the Philippines Food and Drug Administration Advisory No. 2019-152. Please refer to Annex 4 for available photographs.
Bharat Serums and Vaccines Limited, the genuine manufacturer of Equirab, stated:
- They did not manufacture the above listed products.
- The above listed products display labelling and packaging inconsistencies
WHO requests increased vigilance within the supply chains of countries likely to be affected by these falsified products. Increased vigilance should include hospitals, clinics, health centres, wholesalers, distributors, pharmacies and any other suppliers of vaccines.
If you are in possession of the above products, please do not use. If you have used these falsified products, or if you suffer an adverse event having used these products, please seek immediate advice from a qualified healthcare professional, and ensure they report the incident to your local Ministry of Health/National Medicines Regulatory Authorities/National Pharmacovigilance Centre.
All medical products must be obtained from authentic and reliable sources. Their authenticity and condition should be carefully checked. Seek advice from a healthcare professional in case of doubt.
National health authorities are asked to immediately notify WHO if these falsified medical products are discovered in their country. If you have any information concerning the manufacture, distribution, or supply of these medical products please contact rapidalert@who.int
WHO Global Surveillance and Monitoring System
for Substandard and Falsified Medical Products
For further information, please visit: http://covid.comesa.int/medicines/regulation/ssffc/en/