Case study-1: Impacting one billion lives in China: Unblocking the barrier of high prices of HBV medicines towards universal coverage of hepatitis treatment
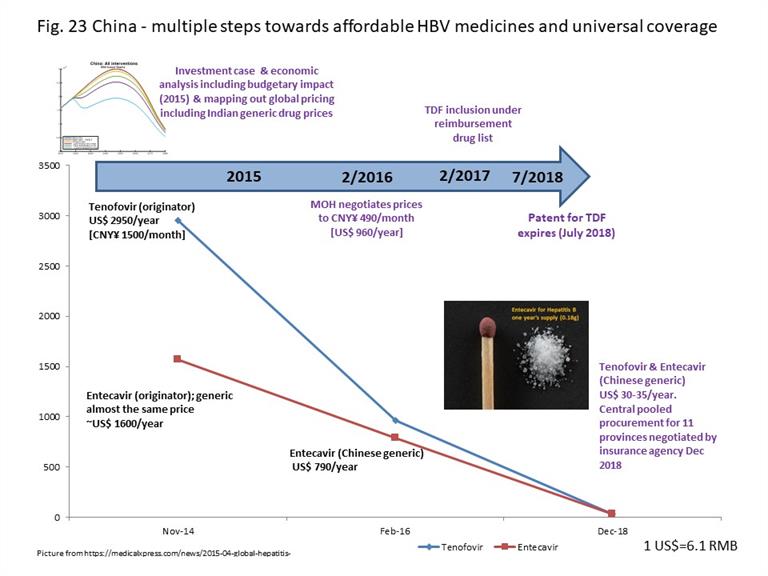
With an estimated 86 million people living with HBV, China has a substantial disease burden. In 2015, the key issue was the high price of HBV medicines. Tenofovir was still under patent protection until July 2018. The cost for use of tenofovir to treat HIV was CNY¥ 120/month (US$240 per year) through national procurement, while the cost to treat patients with HBV was CNY¥ 1 500/month (US$ 2 950 per year), mostly out of pocket. Although entecavir was generically manufactured in China as it no longer had patent protection, generically manufactured medicines often used originator prices to benchmark their retail pricing. Originator entecavir cost CNY¥ 800-1 000 per month (US$ 1 600-2 000 per year), while generic options were priced at about CNY¥ 650-700 per month (US$ 1 300-1 400 per year). Tenofovir and entecavir were not universally and equitably reimbursed through health insurance throughout the country.
Working with WHO and stakeholders, an investment case and economic analyses were conducted to estimate the disease burden, projections of the epidemic, impact on health and on financing health budgets – providing evidence for strategy and policy-making. Sharing drug price information from other countries supported discussions. By February 2016, the National Health Commission had negotiated a reduction in price for tenofovir to CNY¥ 490 per month (US$ 960 per year), and prices of entecavir reduced accordingly. In February 2017, with the updating of the national reimbursement drug list, tenofovir and entecavir were included. At the end of 2018, with patent expiry of tenofovir, the National Insurance Agency negotiated for central pooled procurement supplying 11 provinces (comprising the main treatment burden) as a pilot, reducing the prices for both medicines to US$ 30-35 per year. Extension of this pilot to the whole country in 2019 reduced prices of medicines to US$ 10 per year per person (Fig. 23). Discussions are in progress currently to advocate integration of HBV medicines under the basic essential medicines list which implies free medicines in public sector hospitals. In the longer term, this will facilitate reduction of the lifetime costs of HBV treatment especially for the elderly, who are disproportionately affected with hepatitis B.
Similarly, an investment case and health technology assessment were conducted. Central price negotiations for hepatitis C medicines led by the National Healthcare Security Administration (NHSA) resulted in a more than 85% reduction compared to retail prices, and inclusion of several pangenotypic direct acting antivirals drugs under insurance coverage. Reimbursement for these drugs started 1 January 2020. WHO is working closely with domestic experts, policy think tanks and national programmes to scale up implementation towards the 2030 elimination goal.
Case study-2: Harm reduction in key populations
Malaysia
As of 2018, Malaysia had two national plans addressing the prevention of viral hepatitis transmission among people who inject drugs (PWID), the National Guideline on Methadone Treatment and the National Strategic Plan on Ending AIDS 2016–2030. Both documents address HBV, HCV and HIV counselling and testing, as well as access to needle syringe programmes (NSP) and oral substitution therapy (OST). The Guidelines also include peer-to-peer harm reduction interventions. In Kedah, OST is provided in one hospital, 23 health clinics and 54 private clinics. In 2017, 2477 patients in the state were on OST. At the Klinik Kesihatan (health clinic) Bandar Alor Setar, in Kedah, 900 PWID took advantage of the non-governmental organisation-run NSP. Services such as OST and NSP have also been used to reach out to PWID for annual HIV, HBV and HCV testing. HCV treatment is being piloted in primary care clinics as well as clinics that offer harm reduction services so as to have a “one-stop shop” for care of PWIDs with HIV–HCV coinfection.
Viet Nam
Viet Nam’s National Law on HIV/AIDS Prevention and Control (2005) and Decree 108/2007/ND-CP, which details the implementation of the Law, both provide the legal foundation for comprehensive harm reduction interventions. The Law and Decree support implementation of NSP, OST and condom promotion. These interventions were also included as priorities in the national strategies on HIV prevention and control, and thus have been rapidly scaled up since 2004 with large-scale funding support from external donors. At the end of 2016, 50 766 people were receiving OST, reaching 22% coverage among PWID and in 2016, NSPs distributed approximately 26 million pairs of needles and syringes, equivalent to 148 needles/syringes per PWID per year.
Case study-3: Innovations in hepatitis communications and advocacy – crowdsourcing
Crowdsourcing is the process of having a large group, including experts and non-experts, to solve a problem and then share the solution with the public[1]. Crowdsourcing is an innovative approach increasingly being used in public health. Challenge competitions for innovative product development are a useful means to engage affected communities and the wider public to address social stigma associated with hepatitis infection and encourage testing, while also producing promotional materials for distribution that are of high quality and cost-effective. One example of the use of crowdsourcing was an innovation challenge held in China. In 2017, 13 governmental, nongovernmental, community and international organizations including the Chinese Center for Disease Control and Prevention (China CDC), WHO China and the University of North Carolina Social Entrepreneurship for Sexual Health (UNC-SESH) collaborated on a crowdsourcing challenge contest specifically addressing viral hepatitis. Participants could submit an image or video that encouraged hepatitis testing and addressed social stigma against people living with hepatitis. Contest finalists received recognition and prizes provided by partners during World Hepatitis Day celebrations in Beijing, where the materials were disseminated. A total of 168 images and videos promoting awareness of viral hepatitis were submitted by individuals and organizations as part of the contest. Of note was the fact that 15% of the submissions came from participants identified as chronically infected with HBV or HCV. This demonstrates that such challenges can be a unique opportunity to empower those living with hepatitis to advocate for themselves and offer their unique perspectives on the disease.
[1] World Health Organization and UNICEF, Crowdsourcing in health and health research: a practical guide. 2018, World Health Organization: Geneva.

Case study-4: Understanding and improving testing to treatment cascade
Australia
Funded by the Australian Government Department of Health, the Hepatitis C Mapping Project was developed to improve the national understanding of HCV diagnosis and treatment. Results of the analysis showed that, in 2016, an estimated 227 306 individuals in Australia were living with chronic HCV, and 42 812 (18.8%) had begun receiving treatment. As part of the Project, information on the geographical diversity of HCV prevalence and treatment was also collected to improve understanding of the national disease distribution. This information will be used to assess the progress made towards the goals set in the Australian National Hepatitis C Strategy 2014–2017, and the WHO Global Health Sector Strategy on Viral Hepatitis 2016–2021.
Cambodia
A pilot project in Cambodia, conducted by Médecins sans Frontières (MSF) at the Preah Kossamak Hospital, Phnom Penh, had screened over 25 700 people for HCV by the end of March 2019. The pilot utilized a simplified patient management algorithm requiring five visits, including one physician consultation. Nurses were responsible for the triage of patients and case management until completion of treatment. A total of 11 503 patients initiated DAA-based treatment. Of the 8189 patients who completed treatment, 98% were cured. The model of service delivery was replicated in a rural area in 2018, demonstrating the feasibility of delivering HCV services at the primary care level. Given the high effectiveness and safety of DAAs, task-shifting to nurses can support rapid scaling up of services. The rural sites provided testing for 26 433 people; 1 437 were viraemic, 1 382 initiated treatment and 869 (98% of those who completed treatment) were cured by the end of March 2019.
Mongolia
The Government of Mongolia launched a national programme, The Healthy Liver Programme 2017–2020 (HLP), in May 2017 to accelerate viral hepatitis elimination. The Programme began with nationwide HBV and HCV screening of ages 40–65 in the first year, expanding to ages 15+ in the second year. The Programme’s 2020 targets for HCV are 80%–80%–90% (diagnosed–treated–cured), which are greater than the WHO global and regional action plan 2020 targets of 30% diagnosed and 50% on treatment. The Ministry of Health leveraged national health insurance to cover HBV and HCV testing and treatment costs. In one year of implementation (May 2017 to May 2018) among those aged 40-65-years, results showed that Mongolia was well on its way to achieving its targets. In addition, 47% of the total population aged 40-65 years have been screened for HBV, and 75% of those aged 40-65 years diagnosed as HBsAg-positive are receiving treatment for hepatitis B. Among those living with HCV, 47% of the total population aged 40-65 years screened for HCV and 73% of those aged 40-65 years diagnosed received HCV treatment.




