National reference laboratories and quality-assured testing
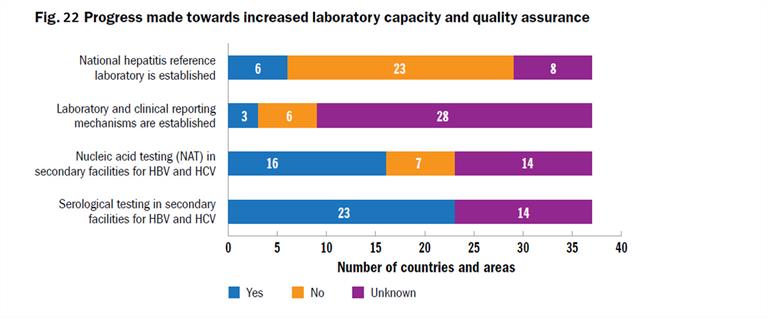
Good-quality laboratory services and quality-assured diagnostic kits are critical for the diagnosis, management and surveillance of viral hepatitis and maintaining a safe blood supply. For these programmes to be effective, test results should be accurate, consistent and delivered in a timely manner. A survey conducted in 2014 among laboratories in the Region showed inadequate capacity for diagnosis and treatment monitoring of viral hepatitis B and C, use of suboptimal or non-quality-assured diagnostics, and inadequate quality assurance systems in several countries. In 2018, this survey was repeated and showed continued gaps in capacities, quality assurance systems and funding compared with laboratory services for HIV and syphilis at the national and regional levels. Only 18 countries provide any official guidance on which tests to use for HBV and HCV diagnosis (Fig. 22).
Access to quality-assured diagnostics
There are limited numbers of WHO-prequalified diagnostic tests for HBV and HCV (see link for the latest update on WHO-prequalified diagnostic kits[1]). There are many diagnostic test kits used in the Region that are not WHO-prequalified or have quality assurance from other stringent regulatory authorities. WHO is working with countries to strengthen regulatory oversight and quality assurance of in vitro diagnostics, quality management systems and evaluation of testing algorithms.
[1] WHO webpage link: https://covid.comesa.int/diagnostics_laboratory/evaluations/PQ_list/en/)




